Contents of Article
- Summary
- What is Salivary Immunoglobulin A (S-IgA)?
- How does exercise impact the immune system?
- What is the relationship between Salivary Immunoglobulin A and physical activity?
- How do you monitor Salivary Immunoglobulin A?
- Is Salivary Immunoglobulin A testing valid and reliable?
- Is future research needed regarding Salivary Immunoglobulin A?
- Who should use Salivary Immunoglobulin A testing?
- Conclusion
- References
- About the Author
Summary
In high-performance sport, monitoring the concentration of salivary immunoglobulin A during intense training periods is often common practice. This is done to assess an athlete’s health and their susceptibility to infection. In recent years, advancements in technology have made it possible to conduct this form of testing both simply and practically.
Though these technologies are extremely useful, their reliability is affected by the ingestion of food, drink, and alcohol prior to testing. This often makes post-exercise testing problematic when athletes desire immediate relief from thirst or hunger. Nevertheless, these technologies have broadened sport scientists’ ability to monitor their athletes and potentially maximise competition performance.

What is Salivary Immunoglobulin A (S-IgA)?
Over the past two decades, there has been an abundance of research conducted to determine the relationship between exercise and immune function (1).
Secretory immunoglobulin A is the main class of antibodies present in the body’s secretory fluids such as saliva, mucus, and tears. Due to its dominance in the immune system of mucus membranes, S-IgA is typically considered the first line of defence from environmental factors (2).
Whilst the full relationship between S-IgA and physical exercise is still not fully understood, enough is known to make assumptions regarding their interaction. For instance, it is understood and commonly agreed that short-term moderate-intensity exercise can improve immune defences, whilst both high-intensity and a lack of exercise can suppress the immune system and are linked to increased upper respiratory tract infections (URTI) (3).
As a consequence, it is theorised that high-performance athletes who have strenuous training regimes are susceptible to contracting URTI as a consequence of a suppressed immune system. Needless to say, exercise scientists have therefore strived to fully understand the relationship between immunity and physical exercise in an attempt to maximise training productivity without exposing athletes to a potential increase in chances of contracting URTIs.
How does exercise impact the immune system?
To date, a vast amount of research has aimed to explain how physical exercise alters the immune system. It is understood that stress induced by physical activity stimulates changes in the lymphatic system, but at present these changes are still not truly understood (2).
The lymphatic system is a one-directional circulatory system that serves to drain, filter and eliminate any forms of harmful substances or diseases from the body (4). It has the ability to neutralise invasive damaging agents such as pathogens. In a healthy individual, the properly functioning immune system comprises lymphatic cells (humoral immunoglobulins) and additional cells outside of the lymphatic system. Correct functioning of these components identifies the proper physiological state of the immune system, and therefore a healthy organism (e.g. human) (2).
Immunoglobulins are a heterogeneous (diverse) group of proteins of the immune system composed of four polypeptide chains: two heavy chains (H) and two light chains (L) connected by disulfide bonds. There are five classifications (isotypes) of immunoglobulins based upon their structural differences in constant heavy chain regions. These five isotypes are IgA, IgD, IgE, IgG, and IgM. The body produces two forms of IgA: serum IgA and secretory IgA. Saliva, tears, and mucus are all examples of secretory IgAs. Although it is believed that secretory IgA complements the neutralisation of harmful pathogens within mucus membranes and stimulates macrophage activation, the primary purpose of this immunoglobulin within serum is still not fully understood (5).
Nevertheless, due to S-IgA dominance in the immune system of mucus membranes, it is typically considered as the first line of defence from environmental factors such as invasive pathogens. It is also believed the concentration of S-IgA varies depending on the current physiological state and physical activity (2). Low concentrations of S-IgA have been linked with an increased risk of contracting URTI (6).
Amongst other factors, the secretion of S-IgA is stimulated by psychological and physical stress levels induced by sport. The secretion and composition of S-IgA is controlled by the activity of the sympathetic and parasympathetic nervous systems. It is believed that stimulation of the autonomous nervous system (sympathetic and parasympathetic) can reduce the amount of saliva and/or inhibit its secretion – ultimately reducing the amount of S-IgA available (7). And as mentioned previously, a reduction in S-IgA is associated with an increased incidence of URTI (6). This information suggests S-IgA may be a useful biological marker to distinguish athletes potentially susceptible to URTI due to strenuous and/or excessive training (8). For this reason, sport scientists often monitor S-IgA amongst high-performance athletes.
What is the relationship between Salivary Immunoglobulin A and physical activity?
It has been reported that the lowest risk of URTI was connected with moderate-intensity exercise, with high-intensity and no exercise linked with the highest risk (1). Likewise, several studies have shown a significant decrease in S-IgA after maximal exertion physical exercise (9, 10, 11) but no change after moderate exercise (12, 13). This information has led experts to identify a possible ‘J-shaped’ relationship between physical activity and the risk of URTI (Figure 1).
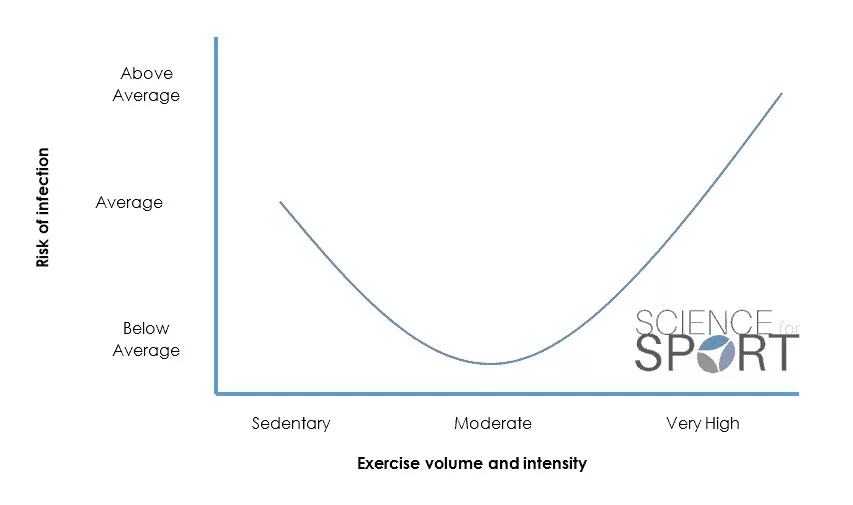
This relationship suggests that both too little and too much exercise may increase an individual’s risk of URTI. Understanding this delicate balance is essential for any sports scientist, particularly those working with elite and professional athletes where training optimisation and availability are so crucial for performance. It also demonstrates the importance of sufficient quantity and quality of recovery periods.
How do you monitor Salivary Immunoglobulin A?
In clinical environments, S-IgA is typically measured using enzyme-linked immunosorbent assay (ELISA) which is currently considered the ‘gold standard’ of salivary biomarker measurement (14). However, this is a laborious process and fails to provide real-time feedback for sports scientists.
As a result, certain companies have developed more practical technologies which are easily accessible, transportable, and can be used post-training or competition to quickly and effectively monitor S-IgA in addition to other biomarkers of physical stress – including Immunoglobulin G (IgG), Cortisol, and Alpha-Amylase. Like with most aspects of technology, with practically often comes a compromise in its validity and reliability.
To date (28/01/16), minimal research (particularly published data) has been conducted on both the validity and reliability of the newly developed practical S-IgA measurement technologies. Nevertheless, one published investigation was conducted in 2015 on the validity and reliability of the Individual Profiling Lateral Flow Device (15 IPRO LTD) in comparison to the ELISA method. The results indicated that the IPRO LTD is both a valid and reliable (r = 0.89, p < 0.001 and CV = 9.4%) measure of S-IgA that can be used as a practical substitute to the ELISA protocol (15).
Is Salivary Immunoglobulin A testing valid and reliable?
Along with the impracticality of using the ELISA method for measuring S-IgA, it is also sensitive to alcohol and recent food and drink intake which can affect the results of the test. This is often an issue following training or competition when athletes desire immediate relief from thirst or hunger.
Although the IPRO LTD is still susceptible to previous alcohol consumption, the IPRO LTD contains buffering agents which may appear to negate the pH variability of recently consumed food or drinks. However, there is no research to date that validates this technology’s ability to reliably measure S-IgA immediately after the consumption of food or drink. This suggests that sports scientists must analyse the data with caution.
Is future research needed regarding Salivary Immunoglobulin A?
To advance the current understanding of the connection between immunity and physical activity, it is imperative to continue investigations into this fascinating relationship. Additionally, in order to progress the current practicality of more portable S-IgA measurement equipment, advancements in the technology must be made. Consequently, the following topics need further exploration:
- Relationships between immunity and physical activity.
- Advancements in current practical technology.
- Impact of food and beverages on the reliability of the IPRO LTD and other likewise models.
- Validity and reliability of IPRO LTD technology on genders and diverse populations (e.g. professional, elite, and sub-elite athletes’).
Who should use Salivary Immunoglobulin A testing?
Measuring S-IgA after training or competition provides the sports scientist with important information regarding the athlete’s susceptibility to contracting URTI. Being able to indicate low S-IgA concentrations should ensure the sports scientist implements adequate recovery and regeneration strategies. For example, additional recovery protocols, enhanced/tailored nutritional programming, immune support supplementation, and increased recovery time. This form of non-invasive assessment can enhance a sport scientist’s physical monitoring profile and enable the improvement of training prescription and optimisation.
Conclusion
Over the past several decades, exercise scientists have established a significant relationship between physical activity and immune system response. As a consequence, advancements in technology have led to useful, reliable, and practical methods of measuring S-IgA after training and competition.
Having the ability to easily measure any potential suppression of the immune system has allowed sports scientists to closely monitor the health of their athletes when under strenuous training regimes. This information should therefore allow sports scientists the ability to optimise training prescription whilst preventing some of the negative impacts associated with intense physical activity.
- Moreira, A., Arsati, F., Cury, P.R., Franciscon, C., Oliveira, P.R., and Arau´jo, V.C. (2009). Salivary immunoglobulin a response to a match in top-level brazilian soccer players. Journal of Strength and Conditioning Research, 23(7), pp.1968–1973. [PubMed]
- Trochimiak, T., and Hübner-Woźniak, E. (2012). Effect of exercise on the amount of immunoglobulin A in saliva. Biology of Sport, 29, pp,255-261. [PubMed]
- Mackinnon, L.T., and Jenkins, D.G. (1993). Decreased salivary immunoglobulins after intense interval exercise before and after training. Medicine and Science in Sports and Exercise, 25, pp.678–683. [PubMed]
- Memmler, R.L., Cohen, B.J., and Wood, D.L. (1996). The Human Body in Health & Disease. 8th Lippincott-Raven: Philadelphia, USA. [PubMed]
- Cunningham-Rundles, C. (2001). Physiology of IgA and IgA deficiency. Journal of Clinical Immunology, 21, pp.303-309. [PubMed]
- Neville, V., Gleeson, M., and Folland, J.P. (2008). Salivary IgA as a risk factor for upper respiratory infection in elite professional athletes. Medicine and Science in Sports and Exercise, 40, pp.1228-1236. [PubMed]
- Proctor, G.B., and Carpenter, G.H. (2007). Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 133, pp.3- 18. [PubMed]
- Fahlman, M.M., and Engels, H.J. (2005). Mucosal IgA and URTI in American college football players: a year longitudinal study. Medicine and Science in Sports and Exercise, 37, pp.374-80. [PubMed]
- Walsh, N.P., Bishop, N.C., Blackwell, J., Wierzbicki, S.G., and Montague, J.C. (2002). Salivary IgA response to prolonged exercise in a cold environment in trained cyclists. Medicine and Science in Sports and Exercise, 34, pp.1632-1637. [PubMed]
- Nieman, D.C., Henson, D.A., and Fagoaga, O.R. (2002). Change in salivary IgA following a competitive marathon race. International Journal of Sports Medicine, 23, pp.69-75. [PubMed]
- Fahlman, M.M., Engels, H.J., Morgan, A.L., and Kolokouri, I. (2001). Mucosal IgA response to repeated Wingate tests in females.International Journal of Sports Medicine, 22, pp.127-131. [PubMed]
- Allgrove, J.E., Gomes, E., Hough, J., and Gleeson, M. (2008). Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. Journal of Sports Science; 26, pp.653-661. [PubMed]
- Johansen, F.E., Braathen, R., and Brandtzaeg, P. (2000). Role of J chain in secretory immunoglobulin formation. Scandinavian Journal of Immunology. 52, pp.240-248. [PubMed]
- Coad, S., McLellan, C., Whitehouse, T., and Gray, B. (2015 – Ahead of Print). Validity and reliability of a novel salivary immunoassay for individual profiling in applied sports science. Research in Sports Medicine. 2, pp.1-11. [PubMed]
- IPRO Interactive | Information on the use of Saliva and Oral fluid. 2015. IPRO Interactive | Information on the use of Saliva and Oral fluid. [ONLINE] Available at: http://www.iprointeractive.com/iga.html. [Accessed 08 December 2015].